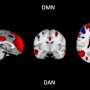
Researchers from **Université Savoie Mont Blanc**, **Radboud University**, and the **University of Oxford’s Wellcome Center for Integrative Neuroimaging** have identified a potential fMRI biomarker that may indicate cognitive decline related to Alzheimer’s disease. The study reveals that the attenuation of the brain’s intrinsic anticorrelation between the default mode network (DMN) and dorsal attention network (DAN) serves as a significant marker, largely independent of tau pathology and cognitive reserve based on education.
Alzheimer’s disease currently lacks a definitive biomarker that can accurately identify the onset of abnormal protein accumulation. This accumulation often leads to network failures and irreversible structural damage. The complexities surrounding the understanding of functional breakdown in Alzheimer’s remain significant, as researchers continue to explore connections among amyloid and tau pathologies, vascular lesions, and neurodegeneration.
A noteworthy aspect of this research is the perspective that **beta-amyloid accumulation** initiates long before clinical symptoms manifest. This suggests that Alzheimer’s disease should be viewed as a continuum, ranging from normal aging to full-blown dementia, prompting scientists to seek insights beyond protein accumulation.
The Dynamics of Brain Networks
The human brain exhibits intricate networks characterized by patterns of cooperation and competition. Recent advancements in resting-state functional MRI have enabled scientists to study these interactions without requiring specific tasks. The DMN and DAN represent two substantial brain systems that typically function in opposition; when one is active, the other is deactivated.
For instance, when an individual engages in daydreaming or self-reflection, the DMN is at play. Conversely, during focused activities such as hitting a baseball or editing text, the DAN takes precedence. Previous studies have established that alterations in resting-state functional connectivity within these networks correlate with cognitive performance in healthy individuals, and disruptions in these patterns are associated with cognitive decline in Alzheimer’s patients.
In this latest study, titled “The intrinsic connectivity between the default mode and dorsal attention networks is an independent fMRI biomarker of Alzheimer’s disease pathology burden,” published in **NeuroImage**, researchers examined MRI and PET amyloid and tau imaging data, alongside cognitive assessments from 182 participants in the **ADNI 3 cohort**. The goal was to determine whether the DMN-DAN anticorrelation aligns with Alzheimer’s pathology burden while testing its independence from tau-driven mechanisms.
Key Findings on Network Interaction
The analysis revealed that participants with high amyloid burden and cognitive impairments exhibited a reduced separation between DMN and DAN signals. This diminished distinction in network activity indicates that cognitive processes may become susceptible to interference, complicating tasks that require focus and information retrieval.
In the full participant cohort, a weaker anticorrelation between the DMN and DAN was consistently linked with lower cognitive scores. Multivariate models—including factors like age, sex, education, and tau burden—highlighted that DMN-DAN connectivity remains a reliable predictor of cognitive performance, accounting for approximately 5% of the variance in scores. Notably, the level of education, serving as a proxy for cognitive reserve, did not alter this relationship.
The implications of this study suggest a shift in how researchers understand the mechanisms of cognitive decline. The findings indicate that network separation—crucial for maintaining distinct inward and outward focus—is least robust in individuals who are amyloid positive and cognitively compromised. Rather than attributing changes solely to a tau-dependent degenerative process, the authors propose a multifocal pattern of disruptions involving various cerebral and extracerebral systems, including vascular issues, sleep disturbances, and stress.
Future research outlined in the study emphasizes the need for longitudinal and interventional investigations to clarify whether network dysfunction occurs before or after abnormal protein accumulation. Additionally, the development of personalized risk models that integrate normative DMN-DAN connectivity patterns, pathology measures, and lifestyle factors is encouraged for a more comprehensive understanding of Alzheimer’s disease.
This research underscores the potential of functional connectivity measures as markers for cognitive dysfunction, offering a new lens through which to view the complexities of Alzheimer’s pathology.
Written by Justin Jackson, edited by Sadie Harley, and reviewed by Robert Egan, this article reflects dedicated journalistic efforts to present independent science reporting. For those who value accurate science journalism, support through donations is appreciated. More information on this study can be found in the work of **Diego-Martin Lombardo et al.** published in **NeuroImage** (2025), DOI: 10.1016/j.neuroimage.2025.121509.