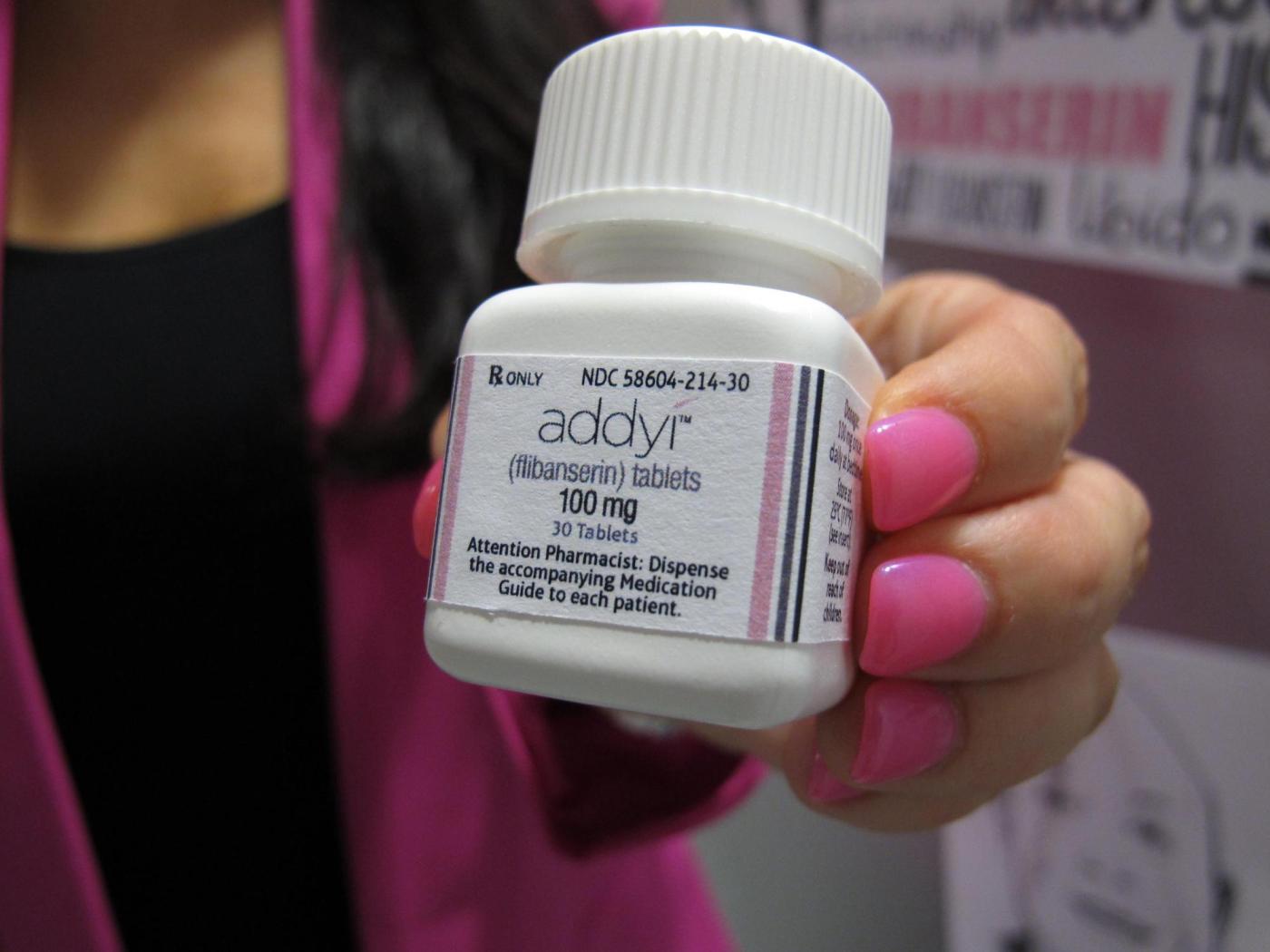
The U.S. Food and Drug Administration (FDA) has expanded the approval of the libido-boosting pill, Addyi, allowing women over the age of 65 who have undergone menopause to use the medication. This announcement, made on Monday, marks a significant shift in the drug’s application, which was initially approved in 2015 for premenopausal women experiencing emotional distress due to low sex drive.
Addyi, produced by Sprout Pharmaceuticals, was originally anticipated to be a major advancement in women’s health. However, its sales have not met expectations due to the presence of side effects such as dizziness and nausea, alongside a serious safety warning regarding alcohol consumption. The FDA has issued a boxed warning, indicating that drinking while taking Addyi can lead to dangerously low blood pressure and fainting.
The approval for older women comes at a time when the medical condition known as hypoactive sexual desire disorder is increasingly recognized. Surveys suggest that this condition affects a significant number of women in the United States. Historically, the diagnosis and treatment of low libido have been complex, particularly as various factors—including hormonal changes after menopause—impact sexual desire.
According to Cindy Eckert, CEO of Sprout Pharmaceuticals, the recent FDA decision reflects a decade-long effort to enhance understanding and prioritization of women’s sexual health. The company announced the update through a press release on Monday, emphasizing the importance of this approval for older women.
Despite the initial promise of Addyi, the drug faced hurdles during its approval process, with the FDA rejecting it twice before its eventual launch. Concerns regarding its modest effectiveness and side effects prompted a lobbying campaign by Sprout and its advocates, framing the need for options for female libido as a matter of women’s rights.
In 2019, the FDA approved a second medication for low female libido, which works through a different mechanism, highlighting the ongoing challenges faced in this area of women’s health.
As awareness of female sexual dysfunction increases, the dialogue surrounding diagnosis and treatment continues to evolve. While some psychologists question whether low sex drive should be classified as a medical issue, the recognition of hypoactive sexual desire disorder marks a significant development in addressing women’s health needs. The FDA’s recent approval of Addyi is a pivotal moment in this ongoing conversation, offering a new option for those affected by this condition.
The AP Health and Science Department receives support from the Howard Hughes Medical Institute’s Department of Science Education and the Robert Wood Johnson Foundation. The AP is solely responsible for all content.