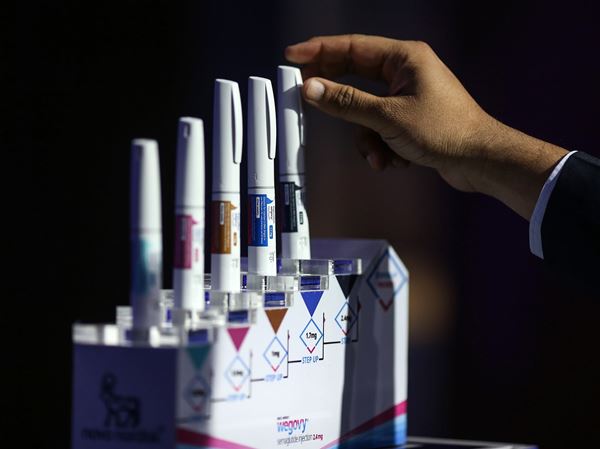
A recent study has revealed that administering lower doses of approved immunotherapy for malignant melanoma can yield better tumor-fighting results while minimizing side effects. Conducted by researchers at Karolinska Institutet, the findings were published in the Journal of the National Cancer Institute. Lead researcher Hildur Helgadottir stated, “The results are highly interesting in oncology, as we show that a lower dose of an immunotherapy drug, in addition to causing significantly fewer side effects, actually gives better results against tumors and longer survival.”
Shift in Treatment Approach in Sweden
Traditionally, the approved dosage for immunotherapy agents such as nivolumab and ipilimumab has been established as the standard treatment. However, due to the serious side effects associated with these drugs, Sweden has started utilizing a regimen with a lower dose of ipilimumab. This particular drug is the most expensive component of the therapy and is linked to the most significant adverse effects. Helgadottir noted, “In Sweden, we have greater freedom to choose doses for patients, while in many other countries, due to reimbursement policies, they are restricted by the doses approved by the drug authorities.”
The study involved nearly 400 patients suffering from advanced, inoperable malignant melanoma, the most aggressive form of skin cancer. Results indicated that the lower dose regimen of ipilimumab was more effective, with 49% of patients responding positively to treatment compared to 37% with the standard dosage. Moreover, the average progression-free survival duration was significantly improved, lasting a median of nine months for those receiving the lower dose, versus just three months for patients on the traditional dosage. Remarkably, overall survival rates also demonstrated a stark contrast, with patients on the lower dose living an average of 42 months compared to 14 months for those receiving the higher dosage.
Addressing Side Effects and Future Implications
The study also highlighted a notable reduction in severe side effects. Only 31% of patients in the low-dose group experienced serious adverse effects, compared to 51% in the traditional group. Helgadottir remarked, “The new immunotherapies are very valuable and effective, but at the same time they can cause serious side effects that are sometimes life-threatening or chronic. Our results suggest that this lower dosage may enable more patients to continue the treatment for a longer time, which is likely to contribute to the improved results and longer survival.”
While the study presents promising outcomes, it is important to note that it is a retrospective observational study. This means that while there were differences between the two treatment groups, the research cannot definitively establish a causal relationship. Even after adjusting for various factors, such as age and tumor stage, the superior outcomes associated with the lower dose of ipilimumab remained evident.
The complete findings of this study, titled “Evaluation of the flipped dose NIVO3+IPI1 in patients with advanced unresectable melanoma,” are set to be published in 2025. For further details, the study can be accessed at doi.org/10.1093/jnci/djaf327.