A recent study conducted by researchers at the University of Rochester has uncovered critical insights into the progression of Batten disease, particularly how sex and age impact its development. Published in the Journal of Neurodevelopmental Disorders, the findings reveal significant differences in brain responses between male and female models of the most common type of Batten disease, known as CLN3.
Batten disease is a rare, inherited disorder that disrupts brain development and function, typically manifesting between the ages of four and seven. Symptoms include vision loss, cognitive decline, movement difficulties, seizures, and speech challenges. As the disease progresses, understanding its trajectory in males versus females has proven complex. Historically, female patients tend to experience a later onset of symptoms and a more rapid progression than their male counterparts.
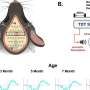
In the study led by Yanya Ding, Ph.D., an alumna of the Neuroscience Graduate Program at the university, researchers utilized a noninvasive technique known as electroencephalography (EEG) to monitor brain activity in mouse models. The study aimed to determine how these models respond to auditory stimuli as the disease progresses.
Ding noted, “Because vision and cognition decline early, it is hard for scientists to track how the disease progresses and develop reliable treatments using standard tests.” The ability to track brain functions in mice could pave the way for innovative approaches to studying potential therapeutics for this challenging condition.
In their findings, researchers discovered that male mice exhibited early auditory problems that tended to improve with age. In contrast, female mice struggled with persistent auditory difficulties. This suggests that both age and sex significantly influence the progression of Batten disease.
The research builds on earlier work by John Foxe, Ph.D., the principal investigator of the Fredrick J. and Marion A. Schindler Cognitive Neurophysiology Lab, who previously identified a biomarker in human CLN3 patients that was applicable to this mouse study. “These findings highlight the importance of tracking brain function over time and support the use of this EEG-based method as a valuable tool for monitoring disease progression and testing new treatments,” Foxe stated.
Kuan Hong Wang, Ph.D., a professor of Neuroscience and co-senior author of the study, emphasized that understanding the different progression patterns in males and females could lead to more personalized therapies. This could also assist in determining the optimal timing for interventions, ultimately improving patient outcomes.
The University of Rochester is recognized as an Intellectual and Developmental Research Center (IDDRC), currently focused on neuromarker discovery in Batten disease. Several potential gene therapies are in advanced stages of development, and the establishment of a translational mouse model may enhance the evaluation of these experimental treatments.
The research team also included co-first author Jingyu Feng, along with Viollandi Prifti, Grace Rico, Alexander Solorzano, Hayley Chang, and Edward Freedman, Ph.D., all affiliated with the University of Rochester Medical Center.
As this field of study progresses, the implications for future treatment strategies are significant, providing hope for more effective interventions for those affected by Batten disease.