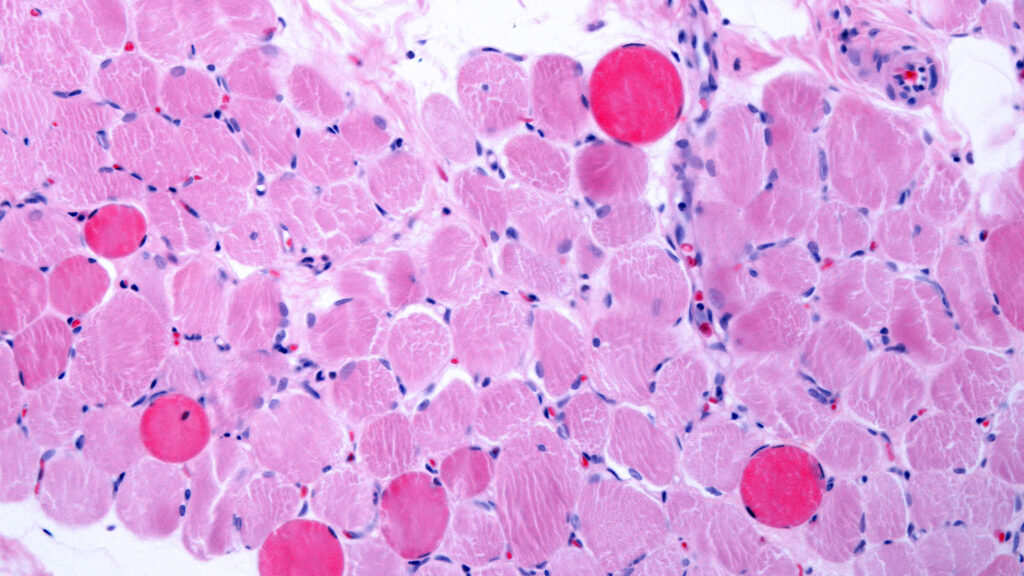
Capricor Therapeutics announced that its cell therapy, known as deramiocel, has significantly improved heart and muscle function in patients suffering from Duchenne muscular dystrophy (DMD). The results come from a Phase 3 study, marking a crucial achievement as the company navigates a challenging regulatory environment following a previous rejection by the Food and Drug Administration (FDA).
The FDA had declined Capricor’s application for deramiocel in July 2023, citing insufficient evidence of effectiveness based on earlier mixed results. The decision was notably influenced by Vinay Prasad, the FDA’s leading regulator for cell and gene therapies, who reportedly overruled staff members who had supported the therapy’s approval.
Positive Results Spark Renewed Hope
Capricor’s latest findings, derived from a larger, placebo-controlled study, present a strong case for the therapy’s efficacy. Linda Marban, the company’s CEO, expressed optimism that these new results would be compelling enough for the FDA to reconsider its earlier stance. The study demonstrated marked improvements in both muscle and heart functions, which are critical markers of health in DMD patients.
Duchenne muscular dystrophy is a severe genetic disorder characterized by progressive muscle degeneration and weakness. Patients with this condition often experience significant challenges in mobility and overall health. The potential approval of deramiocel could provide a much-needed treatment option for those affected by this debilitating disease.
Regulatory Path Ahead
The path to regulatory approval remains complex. Following the FDA’s initial decision, the reception of these new results will be critical in determining the future of deramiocel. Capricor is likely to present this data to the FDA in hopes of overturning the previous rejection.
The company’s announcement comes at a time when advancements in gene and cell therapies are under intense scrutiny, making the stakes particularly high. As the landscape evolves, Capricor’s dedicated research and development efforts could contribute significantly to the treatment of Duchenne muscular dystrophy, offering hope to patients and their families seeking effective therapies.
With the new data in hand, the coming months will be pivotal for Capricor Therapeutics as it seeks to engage with regulatory bodies and advocate for the approval of deramiocel.