Fungal infections pose a significant global health threat, claiming millions of lives annually. Researchers at McMaster University have identified a molecule that could revolutionize treatment options for drug-resistant fungal infections. The compound, known as butyrolactol A, targets Cryptococcus neoformans, a particularly dangerous pathogen that often affects individuals with weakened immune systems.
Cryptococcus infections can manifest with pneumonia-like symptoms and are notoriously resilient against existing treatments. This pathogen primarily affects vulnerable populations, including cancer patients and those living with HIV. The situation is exacerbated by other drug-resistant fungi such as Candida auris and Aspergillus fumigatus, all of which have been classified as priority pathogens by the World Health Organization. Currently, medical professionals have limited treatment options, primarily relying on a drug class called amphotericin. Despite its effectiveness, this drug is often referred to as “amphoterrible” due to its severe side effects.
Identifying a New Hope
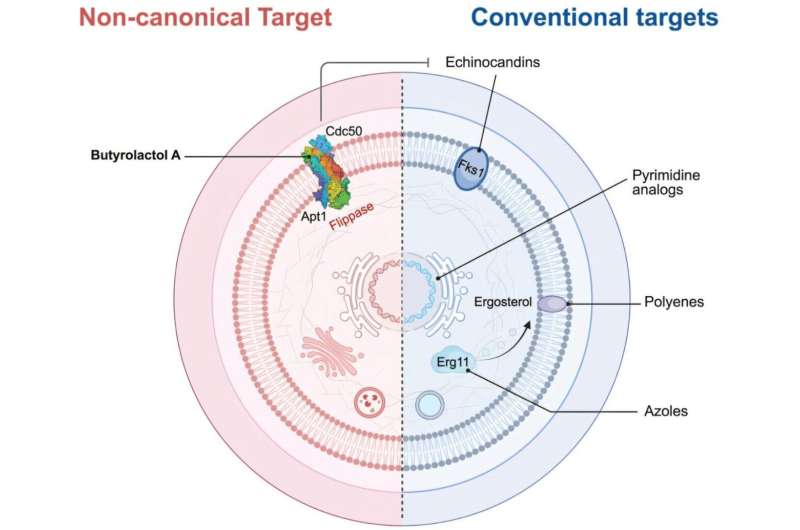
According to Gerry Wright, a professor in McMaster’s Department of Biochemistry and Biomedical Sciences, the options available for treating fungal infections are severely limited. The other two classes of antifungal drugs—azoles and echinocandins—have shown diminishing effectiveness against Cryptococcus. Azoles merely inhibit fungal growth without killing the cells, while echinocandins have become ineffective due to widespread resistance.
In the face of this growing health crisis, researchers are turning to a novel approach involving “adjuvants.” These helper molecules enhance the effectiveness of existing antifungal treatments rather than functioning as standalone drugs. Wright explains that adjuvants make pathogens more susceptible to current medications, providing a potential lifeline for treatment.
In their quest to identify effective adjuvants, Wright’s lab screened a vast collection of chemicals at McMaster. They discovered butyrolactol A, a molecule produced by certain Streptomyces bacteria, which had not been extensively studied since its initial identification in the early 1990s.
Unlocking Mechanisms and Broad Applications
The potential of butyrolactol A was initially met with skepticism, as Wright considered it merely another toxic compound. However, postdoctoral fellow Xuefei Chen urged the team to further investigate its properties. Chen’s dedication led to years of meticulous research that uncovered how butyrolactol A operates.
The team discovered that butyrolactol A acts as a plug, obstructing a crucial protein complex essential for Cryptococcus survival. When this complex is disrupted, the fungus becomes vulnerable to antifungal drugs that were previously ineffective. Wright noted the significance of this finding, emphasizing that it could pave the way for new treatment protocols.
Furthermore, collaborative research with McMaster Professor Brian Coombes has demonstrated that butyrolactol A also enhances the effectiveness of treatments against Candida auris, indicating its broad clinical potential.
The findings, recently published in the journal Cell, have culminated in over a decade of research that originated from a screening in 2014. Wright expressed gratitude for Chen’s perseverance, stating that their efforts have now identified a legitimate drug candidate and a new target for future antifungal therapies.
This discovery represents the second antifungal compound and the third new antimicrobial agent found by Wright’s lab in the past year. With rising rates of drug resistance, the implications of butyrolactol A could be profound, offering hope in the battle against some of the most challenging fungal infections threatening global health.