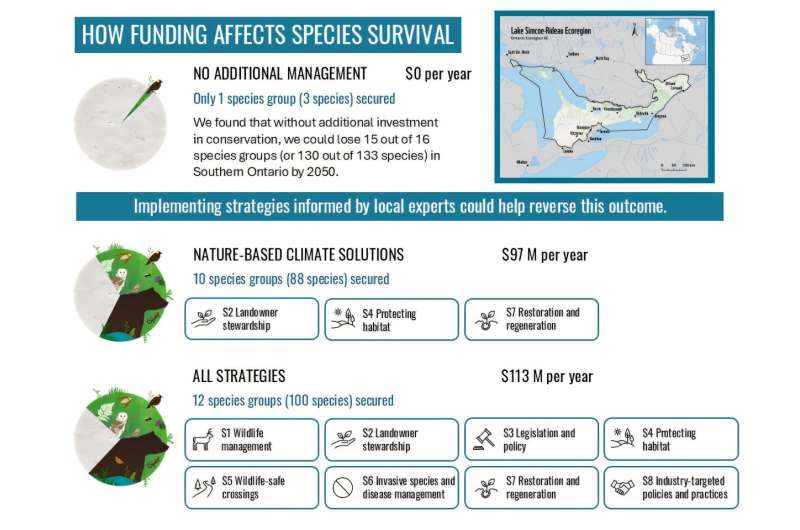
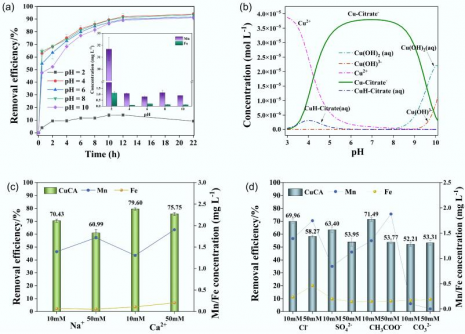
The SISAQOL-IMI consortium has unveiled important guidelines aimed at enhancing patient-reported outcomes (PROs) in cancer clinical trials. Co-led by the European Organization for Research and Treatment of Cancer (EORTC) and Boehringer Ingelheim (BI), the consortium published its findings in a recent article in The Lancet Oncology.
The publication outlines a comprehensive framework for integrating PROs into clinical trials, which are pivotal for assessing patient experiences and treatment impacts. These recommendations were developed through rigorous research and collaboration among experts in the field, aiming to standardize how patient feedback is collected and utilized in cancer research.
Importance of Patient-Reported Outcomes
Patient-reported outcomes are critical as they provide insights into how treatments affect patients’ quality of life. By prioritizing these outcomes, clinical trials can better reflect the real-world impacts of cancer therapies. According to the consortium, incorporating PROs into trial designs can lead to improved patient engagement and more meaningful data on treatment effectiveness.
The guidelines not only emphasize the need for consistent methodologies in gathering PRO data but also highlight the importance of patient involvement in the design of these trials. Engaging patients in the process ensures that the outcomes measured are relevant to their experiences and concerns.
Collaboration and Future Directions
The SISAQOL-IMI consortium is a collaborative effort that brings together various stakeholders in cancer research, including academic institutions, pharmaceutical companies, and patient advocacy groups. This multidisciplinary approach aims to bridge the gap between clinical research and patient needs.
The publication in The Lancet Oncology marks a significant step forward in aligning cancer research with the priorities of patients. The consortium’s efforts are expected to influence future trial designs and regulatory frameworks, ultimately enhancing the relevance and impact of cancer therapies on patient well-being.
As the landscape of cancer treatment continues to evolve, the integration of patient-reported outcomes will likely play a crucial role in shaping more effective and patient-centered approaches in clinical trials. The SISAQOL-IMI consortium’s commitment to advancing these guidelines reflects a growing recognition of the importance of patient voices in cancer research.